*ALPROLIX has been proven to help prevent bleeding episodes in people using prophylaxis.

Looking to stay ahead of bleeds?
ALPROLIX prophy can give you the confidence to help you achieve bleed protection.*

Treating bleeds as they occur?
Just 1 infusion of ALPROLIX stopped most bleeds for adults, adolescents, and kids treating on demand.
ALPROLIX keeps factor IX in the body for longer
ALPROLIX uses Fc Fusion to keep factor IX in the body for longer—more than twice as long as standard half-life (SHL) therapies. This unique design helps ALPROLIX mimic the movement of natural factor IX.
Find your CoRe Manager and connect today
Sanofi Hemophilia Community Relations and Education (CoRe) Managers are here to help people with hemophilia and their families. They share easy-to-understand info about living with hemophilia, including lifestyle tips and treatment choices. Use our CoRe Locator to find someone near you.
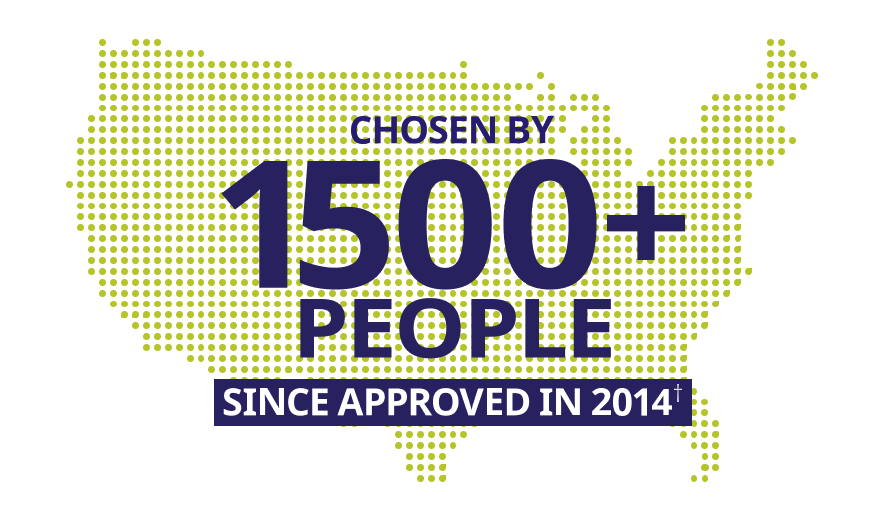

ALPROLIX may help make treatment less burdensome
- Since ALPROLIX is an EHL, it lasts longer in the body, so you may need fewer infusions
- Convenient dosing schedules offer flexibility and freedom to help fit the lifestyles of adults, adolescents, and kids
- ALPROLIX is available in a range of vial sizes that may help make single-vial infusions possible
†Based on records from US specialty pharmacies, third-parties, and internal estimates from May 2014 through July 2024.
“Since starting ALPROLIX...I am grateful I have the protection from bleeds I need.”
—CJ, treating with ALPROLIX since 2014

Important Safety Information and Indication
INDICATION
IMPORTANT SAFETY INFORMATION
INDICATION
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency. Your healthcare provider may give you ALPROLIX when you have surgery.
IMPORTANT SAFETY INFORMATION
Do not use ALPROLIX if you are allergic to ALPROLIX or any of the other ingredients in ALPROLIX.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.
Please see full Prescribing InformationLearn more about Sanofi’s commitment to fighting counterfeit drugs
