47% of people 12 and older who used ALPROLIX on demand in the B-LONG trial switched to a prophy regimen during the B-YOND extension trial


Benefits of ALPROLIX®
on demand
Bleed control across age groups
ALPROLIX stopped most bleeds with 1 or 2 infusions
97%
B-LONG TRIAL
People 12 and up (n=619/636)
92%
KIDS B-LONG TRIAL
Children 11 and under (n=55/60)
94%
PUPs B-LONG TRIAL
PUPs 2 and under (n=80/85)
Most patients rated their response to the first infusion as good or excellent*
8/10
B-LONG TRIAL
People 12 and up (n=513/613)
9/10
KIDS B-LONG TRIAL
Children 11 and under (n=47/53)
9/10
PUPs B-LONG TRIAL
PUPs 2 and under (n=72/79)
Kids B-LONG was a phase 3 study that investigated the safety and efficacy of ALPROLIX in 30 previously treated children with severe to moderately severe hemophilia B. 15 children were 1 to 5 years of age; 15 children were 6 to 11 years of age. All children were treated with individualized prophylaxis.
The PUPs B-LONG study evaluated ALPROLIX efficacy and safety in 33 children 2 years old or less who had never used an infused factor IX product before, sometimes called previously untreated patients, or PUPs. These children (range: 0.1 to 2 years) were studied in a prophylaxis arm (n=28) and an on-demand arm (n=5).
*7 first injections for bleeding episodes in children were not evaluated for response and are excluded from this analysis. Excellent response was defined as abrupt pain relief and/or improvement in signs of bleeding. Good response was defined as definite pain relief and/or improvement in signs of bleeding but possibly requiring another injection in 1 or 2 days.
PUP=previously untreated patient.

effects of ALPROLIX.

manage hemophilia B.
Ready to switch from on demand to prophy?
ALPROLIX may help ease your transition


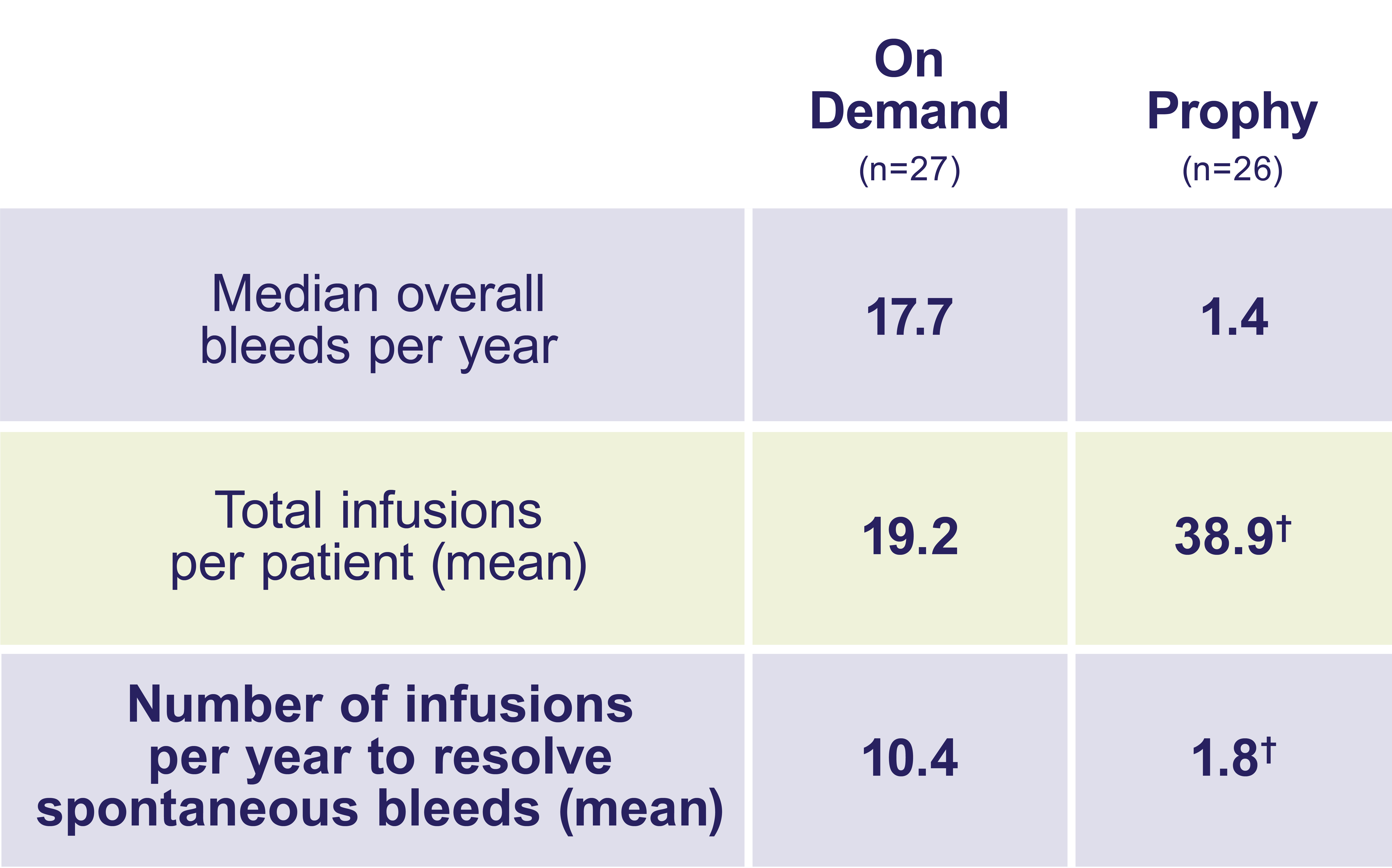
Compared with on-demand treatment, adults and teens on ALPROLIX prophy had fewer bleeds—and fewer infusions to resolve them
ALPROLIX can protect‡ kids 11 and under from bleeds
‡ALPROLIX has been proven to help prevent bleeding episodes in people using prophylaxis.
Prophy=prophylaxis.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.