Frequently Asked Questions
We get these questions a lot
Here are answers to the questions we seem to get the most. If you need more information, your CoRe Manager is a great person to contact or contact Patient Services.
What is ALPROLIX?
ALPROLIX is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency.
Your healthcare provider may give you ALPROLIX when you have surgery.
What prophylaxis dosing options does ALPROLIX offer?
The recommended starting prophylaxis regimens are either 50 IU/kg once weekly, or 100 IU/kg once every 10 days. In children under 12 years of age start at 60 IU/kg once weekly. Dosing regimen can be adjusted based on your individual response.
What is Fc Fusion and how does it help prolong ALPROLIX’s half-life?
Fc Fusion is the science behind ALPROLIX; it helps prolong half-life through the 3-step process below:
-
Bind
- Naturally produced Factor IX flows through the bloodstream and enters cells within your body, eventually breaking it down.
- The Fc portion of ALPROLIX allows it to bind with an Fc receptor already in your body.
-
Redirect
- The binding with Fc receptor redirects ALPROLIX back towards the bloodstream to protect ALPROLIX from being broken down inside the cell.
-
Recirculate
- By using the natural Fc process, ALPROLIX is able to recirculate in the body longer. Eventually ALPROLIX is broken down, but without Fc Fusion, it wouldn’t be able to recirculate in the bloodstream to extend the time you are protected* from bleeds.
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.
How effective is ALPROLIX at treating bleeds in adults and adolescents?
In the B-LONG clinical study, ALPROLIX helped prevent bleeds in people using prophylaxis and controlled bleeds in people treating On Demand. Out of 636 bleeds:
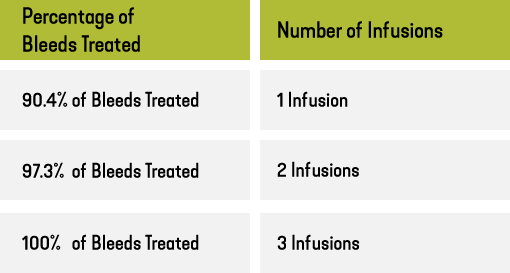
Did prophylaxis treatment with ALPROLIX provide effective protection* from bleeds?
Yes. In 2 completed studies (B-LONG and Kids B-LONG) of people ages 1 to 71 with severe hemophilia B:
- Adults and adolescents who started infusing on a 10-day individualized prophylaxis regimen with ALPROLIX experienced a median annual spontaneous bleed rate (AsBR) of 0.9 bleeds per year. The overall median annual bleed rate (ABR) was 1.4 bleeds per year (n=26).
- Adults and adolescents infusing on a once-weekly prophylaxis regimen with ALPROLIX experienced a median AsBR of 1.04 bleeds per year. The overall median ABR was 3 bleeds per year (n=61).
- Those ages 1 to 5 years experienced a median AsBR of 0 bleeds per year. The overall median ABR was 1.09 bleeds per year and the median joint ABR was 0.
- Those ages 6 to 11 years experienced a median AsBR of 0 bleeds per year. The overall median ABR was 2.13 bleeds per year and the median joint ABR was 1.06.
The dosing regimen can be adjusted based on individual response. You should speak with your healthcare provider to see what dosing regimen would work best for you.
*ALPROLIX has been proven to help patients prevent bleeding episodes using a prophylaxis regimen.
What were the most common drug-related side effects of ALPROLIX?
The most common side effects in previously treated patients were headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system, all of which occurred in 2 people each. Adverse reactions were reported in 14 of 153 (9.2%) subjects treated with ALPROLIX.
In a study of previously untreated patients who were treated with ALPROLIX, 2 of 33 (6.1%) had adverse drug reactions. One person in the study developed a Factor IX inhibitor and the same person developed hypersensitivity. One additional person in the study developed redness to the skin at the injection site.
What should I know about how ALPROLIX is manufactured?
ALPROLIX is a recombinant Factor IX protein.
- ALPROLIX is not derived from human blood and contains no preservatives.
- ALPROLIX undergoes testing in all stages of the manufacturing process: protein production, purification, viral clearance, and formulation.
- To enhance viral safety, the purification process incorporates nanofiltration and purification steps that have been validated for viral clearance.
- Strict standards of quality are maintained during the manufacturing of ALPROLIX.
What vial sizes are available?
ALPROLIX is supplied in single-dose vials labeled 250, 500, 1000, 2000, 3000 or 4000 International Units (IU) per vial of ALPROLIX as lyophilized powder. Liquid diluent comes in a prefilled syringe.
Is there data about inhibitor formation from the ALPROLIX clinical trials?
In the clinical studies of 153 previously treated people, adverse reactions occurred in 14 participants (9.2%). All participants remained in the studies regardless of any adverse reactions. There were zero inhibitors in the 153 previously treated patients using ALPROLIX in the clinical studies.
In the clinical study of 33 previously untreated people, adverse reactions occurred in 2 participants (6.1%). One person (3.0%) in the study developed a Factor IX inhibitor.
Following administration of ALPROLIX, your body could make antibodies against your Factor IX called “inhibitors,” which may stop ALPROLIX from working properly. Your healthcare provider should continue to monitor you for inhibitors with blood tests.
If you have developed an inhibitor with use of another Factor IX therapy, you may be at an increased risk of anaphylaxis with use of ALPROLIX.
What were the study designs?
The safety and efficacy of ALPROLIX have been evaluated in 4 completed studies (B-LONG, Kids B-LONG, B-YOND and PUPS B-LONG) of 186 patients. The B-LONG, Kids B-LONG and B-YOND studies contained 153 previously treated people with severe to moderately severe hemophilia B. There were 126 patients treated for at least 52 weeks and 107 patients for at least 104 weeks.
ALPROLIX was also evaluated in one completed study of 33 previously untreated patients (PUPs) with severe to moderately severe hemophilia B. Median of treatment duration of prophylaxis was 77.5 weeks.
ADULTS AND ADOLESCENTS, 12 YEARS AND OVER
(B-LONG STUDY)
were studied on the once-weekly prophy regimen
- Started with a once-weekly infusion at 50 IU/kg
- Doses were adjusted to maintain desired Factor IX levels
were studied on the individualized prophy regimen
- Given 100 IU/kg every 10 days, and their intervals were adjusted to maintain desired Factor IX levels
were studied to see the efficacy of on demand dosing
- Infusions were given as needed when bleeding episodes occurred
were studied to see the efficacy during surgery
- Doses were adjusted according to the type of surgery
- ALPROLIX was evaluated in 14 major surgeries and 15 minor surgeries
- 4 people in this group did not participate in the other groups
CHILDREN, 1-11 YEARS
(KIDS B-LONG STUDY)
were studied on an individualized prophy regimen
INFANTS AND CHILDREN, LESS THAN 18 YEARS
(PUPS B-LONG STUDY)
were studied on an individualized prophy regimen
were studied to see the efficacy of on demand dosing
What are the important questions to ask my doctor about transitioning to ALPROLIX?
Every question is an important one, especially when you want to learn more about a different treatment. The Talk to Your Doctor section can help you prepare for your next discussion with your healthcare team. It also provides a downloadable guide to take into your appointment. Here are a few questions from our guide for discussions with your healthcare provider:
- How many infusions do I typically have every: Week? Month? Year?
- On average, how many bleeds do I typically have per month?
- How many infusions does it typically take to stop a bleed?
What are the options for covering the cost of ALPROLIX?
Sanofi offers the following financial assistance programs to qualifying individuals:
- Free Trial Plus Program: Offers a 30-day supply at no cost to you. Sanofi Patient Services will review your health insurance information for your coverage of ALPROLIX while you and your doctor decide if this is the right medication for you to continue taking.
- Copay Program: Provides savings on copayment and co-insurance costs.
- Factor Access Program: Helps with gaps in insurance.
- Copay Program not valid for patients utilizing Medicare, Medicaid, VA, DoD, TRICARE®, or similar federal or state programs including any state pharmaceutical assistance programs to pay in part or in full for their prescriptions. Savings may vary depending on patients' out of pocket costs. Free Trial Plus valid only for a patient's first prescriptions and it is limited to one use per patient per product for their lifetime. Free products dispensed through the Free Trial Plus or Factor Access Programs shall not be submitted to any third-party payer, public or private (e.g. private insurance, Medicaid, Medicare, VA, DoD, TRICARE, or similar federal or state programs) for reimbursement. All Programs not valid where prohibited by law. Sanofi reserves the right to modify or terminate the Programs at any time without notice. Program details provided upon registration.
Does ALPROLIX offer a free trial program?
Yes. Sanofi Patient Services will review your health insurance information for your coverage of ALPROLIX while you and your doctor decide if this is the right medication for you to continue taking. Learn more about the Free Trial Plus Program by calling 1-855-MyALPROLIX (1-855-692-5776). Those patients with Medicare or Medicaid or other government healthcare beneficiaries are not eligible for the free trial program.
Not valid for prescriptions covered by or submitted for reimbursement under Medicare, Medicaid, VA, DoD, TRICARE, or similar federal or state programs including any state pharmaceutical assistance programs. Not valid where prohibited by law. Sanofi reserves the right to modify or discontinue the program at any time. Program details provided upon registration.
Where can I get additional supplies, such as needles, gauze, bandages, and tubing?
If you are getting ALPROLIX through a Specialty Pharmacy Provider (SPP), the SPP will provide supplies like these. However, if you are receiving your ALPROLIX through the Factor Access Program or Free Trial Plus Program, contact a MyALPROLIX™️ Coordinator at 1-855-MyALPROLIX (692-5776) to help obtain these supplies for you.
How can I contact a representative?
Sanofi Community Relations and Education, or CoRe, Managers are ready to provide you with resources and education. You can enter your ZIP code to locate your CoRe Manager and email them directly.
What are some of the hemophilia advocacy organizations?
Some advocacy groups are:
- The Coalition for Hemophilia B
(https://www.hemob.org/) - National Hemophilia Foundation
(https://www.hemophilia.org/) - Hemophilia Federation of America
(https://www.hemophiliafed.org/) - World Federation of Hemophilia
(https://www.wfh.org/)
This listing is provided as a resource only and does not constitute an endorsement by Sanofi of any particular organization or its programming. Additional resources on this topic may be available and should be investigated. Sanofi does not review or control the content of non-Sanofi websites. These listings do not constitute an endorsement by Sanofi of information provided by any other organizations.
ENTER YOUR ZIP CODE AND WE'LL FIND THE CoRe NEAREST YOU.
GET MORE ALPROLIX INFORMATION
ALPROLIX 101:
GET THE ESSENTIALS
LEARN MORE
ALPROLIX
PATIENT STORIES
WATCH THEIR STORIES
INDICATION: ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency. Your healthcare provider may give you ALPROLIX when you have surgery.
important safety information and indication
IMPORTANT SAFETY INFORMATION
Do not use ALPROLIX if you are allergic to ALPROLIX or any of the other ingredients in ALPROLIX.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.
INDICATION
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency. Your healthcare provider may give you ALPROLIX when you have surgery.
important safety information and indication
IMPORTANT SAFETY INFORMATION
Do not use ALPROLIX if you are allergic to ALPROLIX or any of the other ingredients in ALPROLIX.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.
INDICATION
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency. Your healthcare provider may give you ALPROLIX when you have surgery.
Please see full Prescribing Information
MANUFACTURED BY
Bioverativ Therapeutics Inc.
Waltham, MA 02451 USA
U.S. License #2078
CLICK HERE TO LEARN MORE ABOUT SANOFI'S COMMITMENT TO FIGHTING COUNTERFEIT DRUGS.
That's okay. We just had to let you know you're about to leave ALPROLIX.com. Thanks for stopping by.
See you again soon.



