bleed and joint bleed protection* for what’s next
Adults and Adolescents on a prophylactic regimen experienced a median of:

ON A PROPHY INDIVIDUALIZED INTERVAL (n=26)

ON A PROPHY FIXED WEEKLY INTERVAL (n=61)

(n=87)
The overall median annual bleed rate (ABR) was 1.4 bleeds per year (n=26). In the B-LONG study, adults and adolescents who started infusing on a 10-day individualized prophylaxis regimen with ALPROLIX experienced a median annual spontaneous bleed rate (AsBR) of 0.9 bleeds per year.
In the same study, adults and adolescents infusing on a once-weekly prophylaxis regimen with ALPROLIX experienced a median AsBR of 1.04 bleeds per year. The overall median ABR was 3 bleeds per year (n=61).
ADULTS AND ADOLESCENTS:

- A median of 0.36 joint bleeds per year on a prophy individualized interval (n=26)
- A median of 1.11 joint bleeds per year on a prophy fixed weekly interval (n=61)
- A median of ~1 spontaneous bleed per year on a prophylactic regimen (n=87)
TARGET JOINT RESOLUTION
100% TARGET JOINT RESOLUTION IN 37 PATIENTS
37/37 patients who had target joints at the baseline of the B-LONG study achieved target joint resolution with ALPROLIX. These subjects had ≥12 months of consecutive follow-up time and did not undergo joint surgery within 12 months of the start of follow-up.
A target joint is defined as a major joint with 3 or more bleeding episodes in a consecutive 3-month period. Target joint resolution is defined as 2 or fewer spontaneous bleeds in a 12-month period.
B-LONG was a phase 3 study that investigated the safety and efficacy of ALPROLIX in 123 previously treated adult and adolescent patients 12 and older with severe hemophilia B. The study included a fixed-interval (weekly) arm (n=63), a fixed-dose (interval-adjusted) arm (n=29), an episodic (On Demand) arm (n=27), and a surgical arm (n=12).
This may help get you started.
Actual healthcare professional being compensated by Sanofi. Not an Actor.
Statement reflects Dr. De Angulo's personal experience and opinion. Individual experiences may vary.
Helping to keep them covered, no matter their age
patients in kids B-LONG and pups B-LONG on a prophylactic regimen experienced a median of:

(kids 1 to 5 years) (n=15)

(pups 0.1 TO 2 years) (n=28)

(kids 6 to 11 years) (n=15)
In the KIDS B-LONG study, which included only previously treated children on individualized ALPROLIX prophylaxis:
- Those ages 1 to 5 years experienced a median annual spontaneous bleed rate (AsBR) of 0 bleeds per year. The overall median annual bleed rate (ABR) was 1.09 bleeds per year and the median joint ABR was 0.
- Those ages 6 to 11 years experienced a median AsBR of 0 bleeds per year. The overall median ABR was 2.13 bleeds per year and the median joint ABR was 1.06.
Kids B-LONG was a phase 3 study that investigated the safety and efficacy of ALPROLIX in 30 previously treated pediatric patients with severe to moderately severe hemophilia B. 15 patients were 1 to 5 years of age; 15 patients were 6 to 11 years of age. All patients were treated with individualized prophylaxis.
The PUPs B-LONG study evaluated ALPROLIX efficacy and safety in people less than 18 years old who had never used an infused factor IX product before, sometimes called previously untreated patients, or PUPs. These children (range: 0.1 to 2 years) were treated with a once-weekly ALPROLIX prophylaxis regimen and experienced an ABR of 0 bleeds.
Alprolix is the only factor IX with two starting prophy dose options
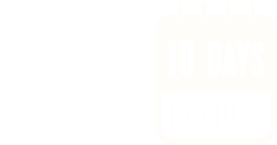
The recommended starting prophylaxis regimens are either 50 IU/kg once weekly, or 100 IU/kg once every 10 days. Dosing regimen can be adjusted based on individual response.
Children under 12 years of age may have higher Factor IX body weight-adjusted clearance, shorter half-life, and lower recovery. A higher dose per kilogram body weight or more frequent dosing may be needed.
More frequent or higher doses may be needed in children <12 years of age, especially in children <6 years of age.
you may be able to extend

In the B-LONG trial, more than half of the people who started on an every-10-day dosing regimen at 100 IU/kg extended to 14 days or longer between infusions.
54% of people in the individualized prophy arm extended to ≥14-day dosing.
The overall median dosing interval on study was 12.5 days. The median interval during the last six months in 26 subjects who were on study for at least nine months was 13.8 days.
The ability to extend was based on individual patient reponse.
INDICATION: ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency. Your healthcare provider may give you ALPROLIX when you have surgery.
important safety information and indication
IMPORTANT SAFETY INFORMATION
Do not use ALPROLIX if you are allergic to ALPROLIX or any of the other ingredients in ALPROLIX.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.
INDICATION
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency. Your healthcare provider may give you ALPROLIX when you have surgery.
important safety information and indication
IMPORTANT SAFETY INFORMATION
Do not use ALPROLIX if you are allergic to ALPROLIX or any of the other ingredients in ALPROLIX.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.
INDICATION
ALPROLIX® [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an injectable medicine that is used to help control and prevent bleeding in people with hemophilia B. Hemophilia B is also called congenital Factor IX deficiency. Your healthcare provider may give you ALPROLIX when you have surgery.
Please see full Prescribing Information
MANUFACTURED BY
Bioverativ Therapeutics Inc.
Waltham, MA 02451 USA
U.S. License #2078
CLICK HERE TO LEARN MORE ABOUT SANOFI'S COMMITMENT TO FIGHTING COUNTERFEIT DRUGS.
That's okay. We just had to let you know you're about to leave ALPROLIX.com. Thanks for stopping by.
See you again soon.



