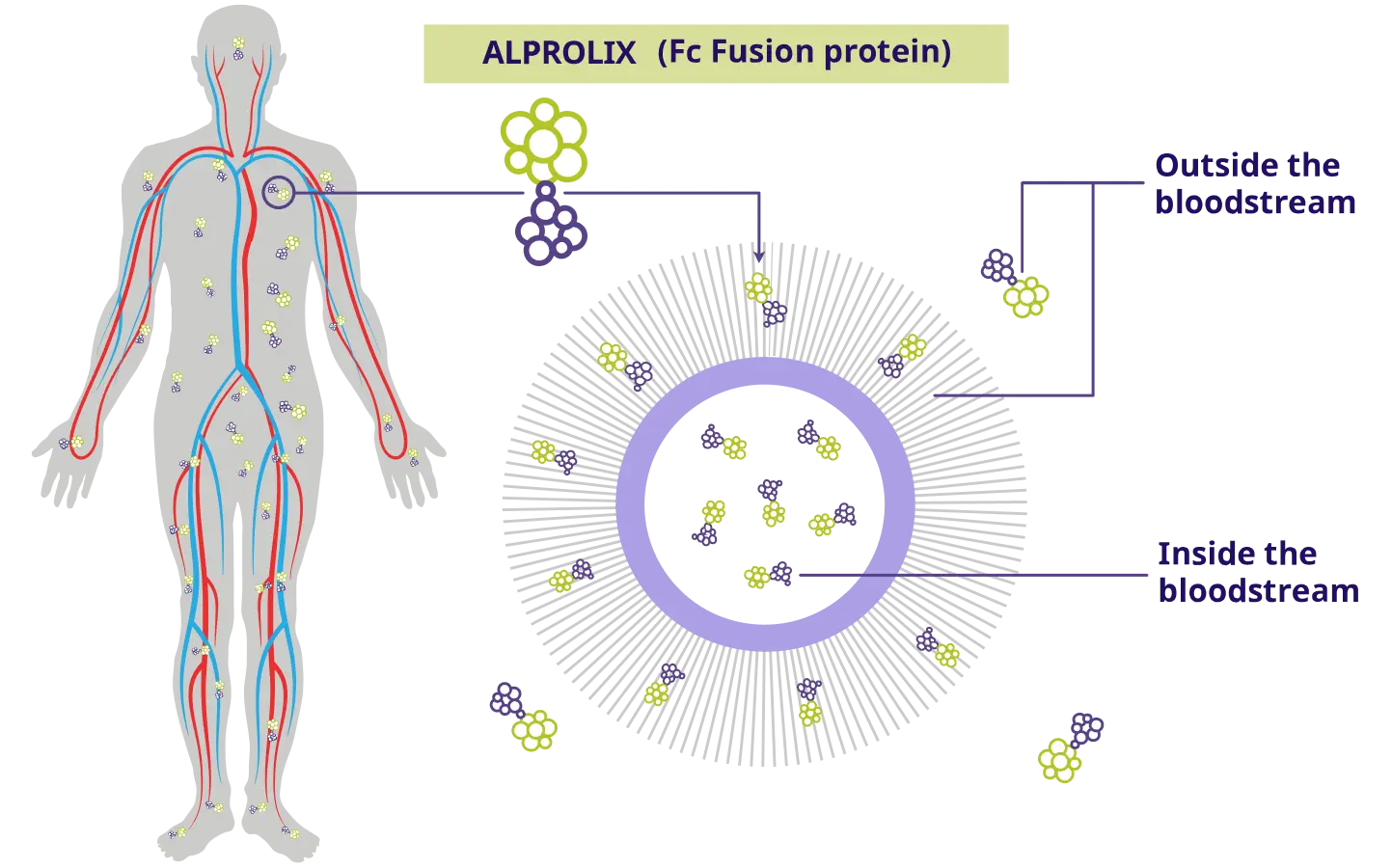
Just like natural factor IX, ALPROLIX leaves the bloodstream and takes a natural pathway out to tissues, muscles, or joints.
Because of this movement, traditional lab work may not be enough to determine treatment success.
Monitoring outcomes such as bleed control or prevention will be important for your doctor to know how ALPROLIX is working for you.


How ALPROLIX® works
ALPROLIX helps people with hemophilia B control or prevent bleeds by replacing the missing factor IX in the body.
ALPROLIX uses Fc Fusion to keep factor IX in the body for longer
Fc Fusion: Factor IX + Fc molecule fuse to make ALPROLIX

Fc Fusion extends the half life of ALPROLIX
The longer half life of ALPROLIX makes it an extended half-life (EHL) therapy. It can stay active in your body longer than standard half-life (SHL) therapies.
The most important measure of your success is number of bleeds
While your doctor may request lab work to measure your factor activity, monitoring bleeds (and bleed resolution) will be critical to measuring your success with ALPROLIX.
Because ALPROLIX works like natural factor IX, success should be measured by outcomes, like bleed rates



*ALPROLIX has been proven to help prevent bleeding episodes in people using prophylaxis.
Tell your healthcare provider if you have or have had any medical problems, take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines, have any allergies and all your medical conditions, including if you are pregnant or planning to become pregnant, are breastfeeding, or have been told you have inhibitors (antibodies) to Factor IX.
Common side effects of ALPROLIX include headache, abnormal sensation in the mouth, and pain in your side with blood in your urine, which may be a sign of clot formation in the urinary collecting system.
Allergic reactions may occur with ALPROLIX. Call your healthcare provider or get emergency treatment right away if you have any of the following symptoms: hives, chest tightness, wheezing, difficulty breathing, or swelling of the face.
Redness to the skin at the injection site may also occur.
ALPROLIX may increase the risk of formation of abnormal blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider or seek emergency care if you have symptoms of a possible abnormal blood clot, which may include: chest pain, difficulty breathing, unexpected swelling of an arm or leg with or without pain or tenderness.
Your body can also make antibodies called "inhibitors" against ALPROLIX, which may stop ALPROLIX from working properly.
These are not all of the possible side effects of ALPROLIX. Talk to your healthcare provider right away about any side effect that bothers you or does not go away, or if bleeding is not controlled using ALPROLIX.